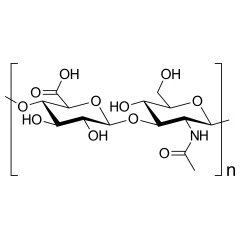
Name: Sodium Hyaluronate
Cas no.: Cas 9067-32-7
Grade: European Pharmacopoeia
Origin: Fermentation from Streptococcus
Molecular weight: Different ranges available, please request at [email protected]
Prices are available upon request with our account manager for this product: [email protected]
**Pricing disclaimer
Sodium hyaluronate is the sodium salt of hyaluronic acid, a glycosaminoglycan found in various connective tissue of humans.
Sodium hyaluronate compliant with the latest European pharmacopoeia. The European Pharmacopoeia (Ph. Eur.) is a compendium of quality standards for pharmaceutical substances and medicinal products, recognized as legally binding across the European Union (EU) and European Economic Area (EEA). It provides specifications for the identity, purity, strength, and quality of substances used in medicines, including active pharmaceutical ingredients (APIs) and excipients, as well as guidelines for dosage forms and analytical methods.
Additional testing of the raw materials according to the ICH Q3C residual solvents or ICH Q3D Elemental impurities is possible upon request.